Generic drugs are essential to global healthcare, making treatments more affordable and accessible to the audience. They account for over 90% of prescriptions in the U.S. market and about two-thirds in the European market. However, developing a generic isn’t just about copying a brand drug; it requires strict bioequivalence (BE) studies to prove the generic is just as safe and effective as the original.
What are Bioequivalence Studies?
Bioequivalence studies are the cornerstone of generic drug development. These studies compare the rate and extent of absorption of the generic drug with that of the Reference Listed Drug (RLD).
- A test product is considered to be bioequivalent to a reference product if the 90% confidence interval (CI) of the geometric mean ratio (GMR) of AUC (area under the concentration vs. time curve) and Cmax (maximum concentration) between the test and reference fall within the limits of 80 to 125%.
- This ensures patients receive the same therapeutic benefit whether they use the branded or generic version.
Generics manage to save the U.S. healthcare system over a billion dollars annually while maintaining high standards of safety and efficacy.
Challenges in Designing and Executing Bioequivalence Studies
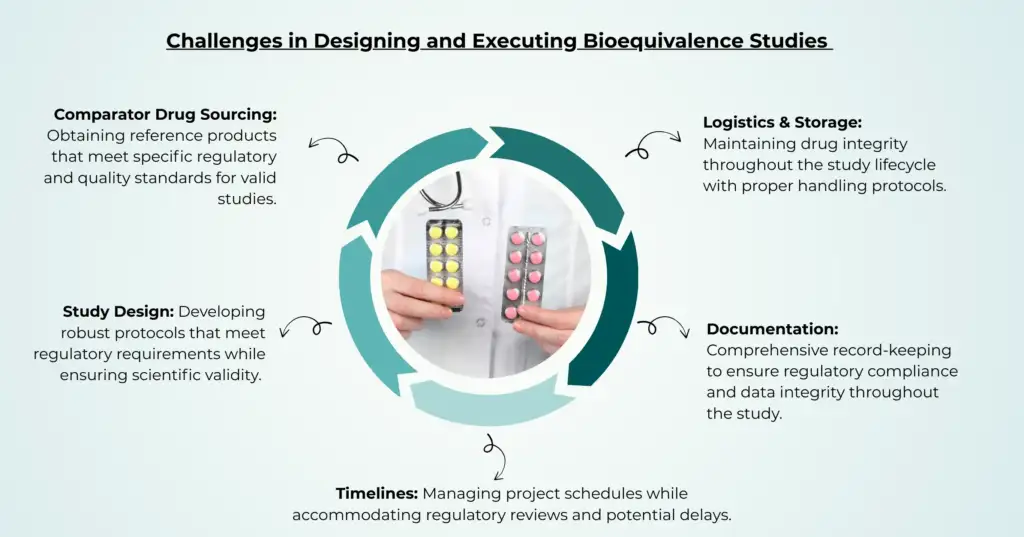
Designing and executing bioequivalence studies has its own set of challenges. Companies face multiple hurdles in planning and executing bioequivalence studies. From sourcing authentic reference drugs to maintaining strict regulatory compliance, every step poses unique challenges. Here are five critical factors that can make or break a successful bioequivalence study.
Study Design
Crafting robust protocols that balance scientific validity with regulatory compliance is critical. Factors like subject selection, dosing regimen, and crossover design must be optimized to minimize variability and ensure reliable outcomes.
Comparator Drug Sourcing
Procuring authentic reference drugs from approved markets is often challenging due to availability issues, regional restrictions, or batch size requirements. Any compromise here can undermine the study’s credibility.
Logistics & Storage
Maintaining drug integrity requires strict control over transportation, cold-chain management, and storage conditions. Delays in shipment or customs clearance can disrupt study timelines and drug quality.
Documentation
Comprehensive records, including study protocols, drug accountability, and adverse event reports, are vital for regulatory compliance. Missing or inconsistent data can delay approvals and trigger audit concerns.
Timelines
Coordinating subject recruitment, regulatory approvals, and drug availability while preparing for potential delays is complex. Strong project management and risk planning are essential to keep studies on track.
Failing to address these challenges effectively can result in delays, repeat studies, or regulatory rejection.
How Spring Bio Solution Supports Generic Players
At Spring Bio Solution, we specialize in RLD and comparator drug sourcing, one of the most challenging yet critical aspects of BE studies. Our expertise helps generic manufacturers:
- Access authentic reference drugs with full pedigree support, proof of purchase, and chain-of-custody documentation.
- Maintain proper storage, labeling, and GCP/GMP compliance throughout the trial supply chain.
- Overcome availability issues by leveraging our global sourcing network
- Reduce the risk of counterfeit or compromised products through advanced track and trace solutions
- Seamless clinical trial logistics & regulatory support
By partnering with us, generic developers can focus on conducting clinical trials, while we handle the complexities of sourcing, logistics, and regulatory compliance.
Conclusion
The future of healthcare depends not only on new drug discoveries but also on the trust patients place in generics. By strengthening the bridge between innovation and accessibility, bioequivalence studies and reliable trial supply partners help ensure that every patient—regardless of their geographical location—receives safe, effective, and affordable medicine.
If you’re a generic drug manufacturer looking for reliable RLD sourcing and support, let’s connect and explore how we can help you deliver affordable medicines to the world.