The year 2024 proved to be a turning point for pharmaceutical innovation. From oncology to metabolic and respiratory disorders, a new wave of therapies is redefining treatment paradigms. These therapies are creating significant market opportunities for generic challengers.
Ten regulatory agencies-approved drugs are already being called the next generation of blockbusters, with each projected to surpass $1 billion in annual sales by 2029. But beyond their commercial promise, these molecules also serve as a roadmap for strategic Para IV filings and smarter, data-driven market-entry decisions.
Why These Approvals Matter for Para IV Filers
Every blockbuster starts with a ‘New Chemical Entity’ designation that grants five years of exclusivity. But for generics, the real window of opportunity lies in the NCE-1 date, one year before that exclusivity expires.
Tracking these NCE and NCE-1 timelines allows companies to:
- Predict Para IV filing windows with precision
- Identify high-value targets with billion-dollar potential
- Plan formulation, sourcing, and regulatory strategies proactively
In a space where timing defines success, missing a single NCE-1 date could cost you years of potential market share.
Spotting the Next Billion-Dollar Opportunity
With one single login, users can access an NCE-1 intelligence platform that provides:
- Molecule name and innovator
- Dosage form and strength
- Therapeutic use
- NCE and NCE-1 dates
- Revenue, CAGR %, and projected sales
This real-time intelligence helps R&D, regulatory, and business development teams stay ahead of the curve, aligning product selection with high-growth markets and upcoming exclusivity expirations.
Top 10 Drug Approvals of 2024: Mapping Smart Para IV Opportunities Ahead
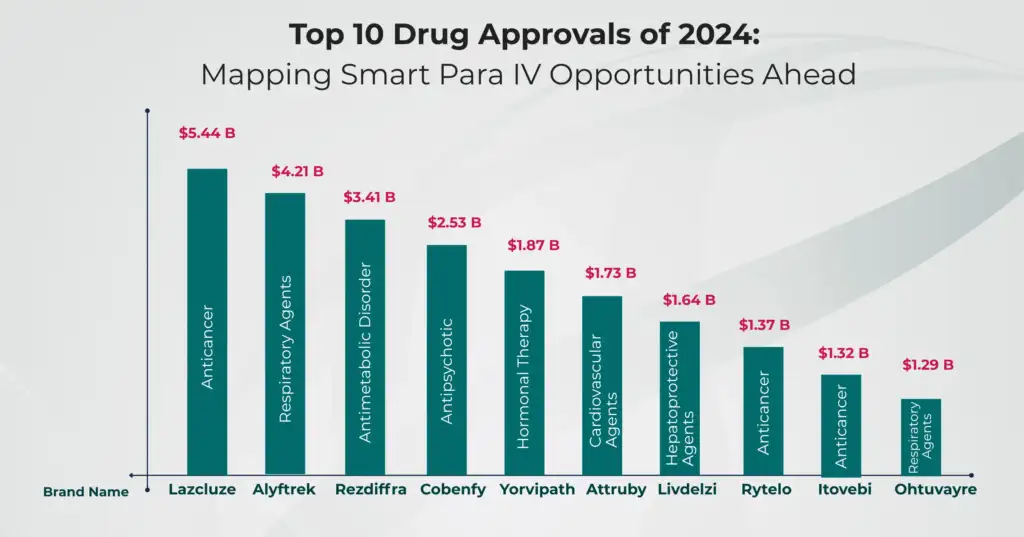
The Future Belongs to the Fast Movers
Innovation moves fast, and so does competition. As the 2024 drug class begins to shape the next decade of pharma growth, companies that combine intelligence with timing will lead the Para IV race.
With actionable insights from NCE Grid, it’s no longer about watching market trends; it’s about anticipating them.
About Spring Bio Solution
At Spring Bio Solution, we are the trusted force behind over 12,000 successful bioequivalence projects and 1,000+ global clinical trials. Backed by over fifteen years of experience and deep market intelligence, we specialize in strategic comparator sourcing, end-to-end clinical trial supply chain management, and regulatory-compliant drug distribution.
FAQs
How does tracking NCE and NCE-1 dates help in Para IV planning?
NCE (New Chemical Entity) exclusivity protects a drug molecule for five years after approval. Still, the NCE-1 date, one year before exclusivity expires, marks the first chance for generic challengers to file Para IV applications. By monitoring these timelines, companies can strategically position themselves for first-to-file advantages and faster market access.
What is the NCE-1 date?
The NCE-1 date is often the first opportunity for a generic drug manufacturer to submit an ANDA referencing a branded drug with NCE exclusivity. It is therefore critical to pursue the 180-day marketing exclusivity granted to a first-filed ANDA.
What is New Chemical Entity Exclusivity?
New Chemical Entity exclusivity is a five-year period granted by the FDA to a new drug that does not contain a previously approved active moiety.
What is the difference between NCE and NCE-1?
The NCE-1 drug represents a slight modification of an existing approved drug. The term “minus 1” implies that the New Chemical Entity (NCE) is only one small change away from an already FDA-approved and marketed drug.





